MEMORIAL SEMINAR
Dedicated to the memory of
Valerii Borisovich Smirnov

Seminar program:
1. A.Vasilyeva
«Femtosecond laser induced formation of silver nanoparticles in Ag2O-P2O5 glassy system.»
2. M.Pyshnyak
«Excited state dynamics of substituted tetraphenylporphyrins and their adducts with fullerene.»
October, 15 2014, 13-00,
RC «Optical and laser materials research» SPSU,
Ulyanovskaya st., 5
 |
From 1 to 3 October on the basis of the Center for Optical and Laser Materials Reseach with the support of G-RISC (German-Russian Interdisciplinary Science Center) and the Russian Chemical Society the lectures on electrochemistry will be held by Professor of Faculty of Chemistry, Friedrich-Alexander University, Nuremberg, Germany (Friedrich-Alexander-Universität Erlangen-Nürnberg) Julien Bachmann. Everyone is welcome! Information about the program, time and venue will be announced later. |
|
October 1, Turning lead into gold: Materials and nanostructures in electrochemical energy conversion. Institute of Chemistry, 315 auditory, 17:30 October 2, Exchange phenomena at elongated structures, from macro to nano -- optimizing energy conversion devices based on their geometry, RC OLMR, 209 auditory, 12:00 October 3, Aspects of catalysis and electrocatalysis: materials and geometries (lecture within the frame of Russian chemical society). Institute of Chemistry, 03 auditory, 17:15 |
|
V.V. Sokolov, A.Yu. Ivanov, M.S. Avdontseva, A.A. Zolotarev
«Stereochemistry and NMR Spectra of Some Tricyclic Condensed Thiazolidine Derivatives with a Bridgehead Nitrogen Atom»
Chem. Hetercycl. Compd. 2014, 50, 550-556
DOI: 10.1007/s10593-014-1506-3

The configuration of a series of tricyclic condensed thiazolidines with a bridgehead nitrogen atom, for which erroneous data had been published, was determined by X-ray structural analysis and NMR spectroscopy.
V.A. Rassadin, E. Nicolas, Y. Six
«Ti(OiPr)4/nBuLi: an attractive reagent system for [2+2+2] cyclotrimerisation reactions»
Chem. Commun. 2014, Advance Article
DOI: 10.1039/C4CC02698E

A convenient method for the [2+2+2] cyclotrimerisation of alkynes using Ti(OiPr)4/nBuLi is presented. Homotrimerisation of arylacetylenes proceeds within minutes with excellent regioselectivity. Moreover, the intermolecular construction of ABB heterotrimers can be achieved selectively from two different alkynes with similar electronic properties. The method is also suitable for the synthesis of pyridines.
J. Malinina, T.Q. Tran, A.V. Stepakov, V.V. Gurzhiy, G.L. Starova, R.R. Kostikov, A.P. Molchanov
«[3+2] Cycloaddition reactions of arylallenes with C-(N-arylcarbamoyl)- and C,C-bis(methoxycarbonyl)nitrones and subsequent rearrangements»
Tetrahedron Lett. 2014, 55, 3663-3666
DOI: 10.1016/j.tetlet.2014.04.107
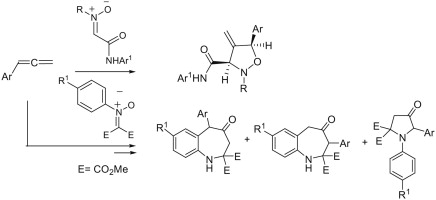
The first example of 1,3-dipolar cycloaddition reactions of nitrones with arylallenes is described. C-Carbamoylnitrones react with the C1single bondC2 double bond of arylallenes affording the corresponding 4-methyleneisoxazolidines in good yields. N-Aryl-C,C-bis(methoxycarbonyl)nitrones usually gave rearranged products: mixtures of 4,5-dihydro-4-oxo-1H-benzo[b]azepine-2,2(3H)dicarboxylates and 4-oxo-1,5-diarylpyrrolidine-2,2-dicarboxylates.

