Within the Mendeleev-2014 conference we held the Open Doors Day. Excursionists looked at our equipment, got acquainted with basics of NMR and working principles of the Center, received promotional materials.
Dear Users,
In the Center we have developed a unique technique for measuring NMR spectra of carbon 12C. In connection with this since tomorrow measurements application of 13C will not be accepted. Note that samples for 12C spectra measurement must be prepared in special U-shaped square tubes. Applications for the new method of measurement shall be approved by all members of the Center.
You can download the application form here.
On Wednesday, April 2, 2014 during the conference “Mendeleev-2014″ there will take place an open day in our Center. A tour around the Center is scheduled to start at 15:00 to 16:30 for all interested conference participants.
Alena S. Pankova, Mikhail A. Kuznetsov
«Synthesis and thermal transformations of spiro-fused N-phthalimidoaziridines»
Tetrahedron Letters, 2014, In Press
DOI: 10.1016/j.tetlet.2014.03.014
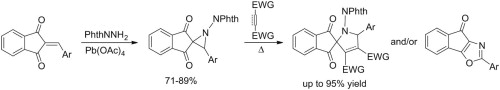
Oxidation of N-aminophthalimide in the presence of 2-arylideneinden-1,3-diones with electron-withdrawing substituents gives the corresponding 3-aryl-1-phthalimidospiro[aziridine-2,2’-indene]-1’,3’-diones in good yields. Heating these aziridines with standard dipolarophiles (N-phenylmaleimide, dimethyl acetylenedicarboxylate, maleate and fumarate) leads, in most cases, to spiro[inden-2,2’-pyrrole] derivatives as products of 1,3-dipolar cycloaddition of the intermediate azomethine ylides with up to 70-95% yields in the case of N-phenylmaleimide. As is typical for 2-acylaziridines, the competing rearrangement into 2-aryl-4H-indeno[2,1-d][1,3]oxazol-4-ones prevails for less active dipolarophiles. Increasing the electron-releasing properties of the 3-aryl ring allows the observation of the push-pull effect of electron-donating and electron-withdrawing substituents on the ease of the three-membered ring-opening.
- 12.03.2014 Visiting rectors
- 07.03.2014 Dalton Transactions
- 07.03.2014 Chemistry of Heterocyclic Compounds
- 06.03.2014 Announcing seminars on March 13th and 20th, 2014
- 10.02.2014 A scientific talk will be held at 12.00 by professor Gerd Leuchs, the director of Max Planck Institute for science of light




