|
|
Elena Kurenkova has attended the course “Basics of the pulsed-field-gradient NMR for diffusion studies” in the Royal Institute of Technology, Stockhol, and got the certificate. |
- Details
- Hits: 1421

- Details
- Hits: 1317
- Details
- Hits: 1429
In the VII Symposium «Nuclear Magnetic Resonance in Chemistry, Physics and Biological Sciences» that took place on 25-28 of September 2012 in Warsaw, Poland, we presented a talk:
Peter Tolstoy, Benjamin Koeppe, Jing Guo, Svetlana Pylaeva, Elena Tupikina, Gleb Denisov, Hans-Heinrich Limbach
«Effects of Solvation Shell Structure and Dynamics on Geometry and NMR/UV-vis Spectra of H-bonded Complexes»
and two posters:
1) Elena Tupikina, Peter Tolstoy, Sergey Smirnov, Nikolai Golubev, Gleb Denisov «Hydroxyphenyl Benzimidazole as Catalyst for Hydrolysis Reaction: Serine Protease Active Site Model»
2) Svetlana Pylaeva, Peter Tolstoy, Benjamin Koeppe, Christoph Allolio, Daniel Sebastiani, Gleb Denisov «Distribution of Hydrogen Bond Geometries and Spectroscopic Parameters Due to the Solvent-Solute Interactions Studied by Means of Computer Simulations».
«Effects of Solvation Shell Structure and Dynamics on Geometry and NMR/UV-vis Spectra of H-bonded Complexes»
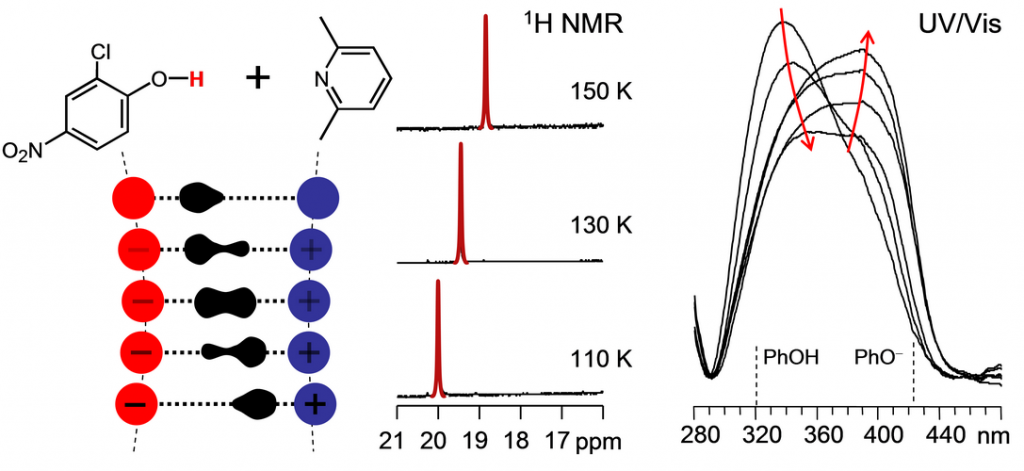
Geometries of strong H-bonded complexes in solutions are quite susceptible to steric hindrances, local electric fields and weak solvent-solute interactions. Thermal fluctuations within the complex as well as fluctuations of the solvent molecules and couterions (for charged systems) in the solvation shell lead to a constant re-arrangement of the H-bond geometry. As a result, a distribution of rapidly interconverting solvent configurations – “solvatomers” – is created, with the lifetime usually not exceeding 10-9 s. This process is fast on the NMR time scale and NMR signals of solvatomers are averaged out into a single narrow peak. In contrast, the time scale of optical spectroscopy is much shorter and spectral bands of individual isomers can be observed individually or as parts of the inhomogeneously broadened band.
In this work we summarize the experimental results obtained over the last couple of years for neutral (OHN) and anionic (OHO-) strong H-bonded complexes dissolved in polar aprotic solvents (CDF3/CDF2Cl, CDCl3, CD2Cl2) by means of low-temperature NMR and combined NMR/UV-Vis spectroscopy. Main types of interacting partners include carboxylic acids, phenols any pyridines. Using so called “hydrogen bond correlations” we are able to interpret spectral features in terms of interatomic distances.
Analyzing the H-bond geometries we reconstruct an “adiabatic” proton transfer pathway and attempt to identify the essential solvent-solute interactions which could explain the experimentally observed trends. Example in the Figure shows1H NMR and UV-vis data for a 2-chloro-4-nitrophenol complex with 2,6-lutidine as well as resulting qualitative pathway for the proton transfer. The roles of solvent-solute dipole-dipole interactions, solvent ordering around the localized charges, counterion effects and weak H-bonding with the acidic CH groups of organic solvents are discussed.
- Details
- Hits: 1279
B. C. K. Ip, I. G. Shenderovich, P. M. Tolstoy, J. Frydel, G. S. Denisov, G. Buntkowsky and H.-H. Limbach,
«NMR Studies of Solid Pentachlorophenol-4-Methylpyridine Complexes Exhibiting Strong OHN Hydrogen Bonds: Geometric H/D Isotope Effects and Hydrogen Bond Coupling Cause Isotopic Polymorphism»
J. Phys. Chem. A 2012, ASAP.
DOI: 10.1021/jp305863n.

Abstract:
We have studied the hydrogen bond interactions of 15N labeled 4-methylpyridine (4-MP) with pentachlorophenol (PCP) in the solid state and in polar solution using various NMR techniques. Previous spectroscopic, X-ray, and neutron crystallographic studies showed that the triclinic 1:1 complex (4-MPPCP) exhibits the strongest known intermolecular OHN hydrogen bond in the solid state. By contrast, deuteration of the hydrogen bond gives rise to the formation of a monoclinic structure exhibiting a weaker hydrogen bond. By performing NMR experiments at different deuterium fractions and taking advantage of dipolar 1H–15N recoupling under combined fast MAS and 1H decoupling, we provide an explanation of the origin of the isotopic polymorphism of 4-MPPCP and improve previous chemical shift correlations for OHN hydrogen bonds. Because of anharmonic ground state vibrations, an ODN hydrogen bond in the triclinic form exhibits a shorter oxygen–hydron and a longer oxygen–nitrogen distance as compared to surrounding OHN hydrogen bonds, which also implies a reduction of the local dipole moment. The dipole–dipole interaction between adjacent coupled OHN hydrogen bonds which determines the structure of triclinic 4-MPPCP is then reduced by deuteration, and other interactions become dominant, leading to the monoclinic form. Finally, the observation of stronger OHN hydrogen bonds by 1H NMR in polar solution as compared to the solid state is discussed.
- Details
- Hits: 1159
On the VVV-2012 conference that took place in Novosibirsk on the 15-20 of July 2012 was presented a talk
P. M. Tolstoy, B. Koeppe, J. Guo, S. A. Pylaeva, G. S. Denisov, H.-H. Limbach, “Reaction Pathways of Proton Transfer in Anionic OHO Hydrogen Bonded Complexes”.
- 14.07.2012 NMRCM-2012 conference
- 10.07.2012 New article in J. Phys. Chem. A is published
- 18.06.2012 New article in PCCP is published
- 16.05.2012 Russian-Finnish Conference on Technology Transfer, Entrepreneurship and Research Infrastructure Management
- 28.03.2012 Our article made it to the cover of the Journal of Physical Organic Chemistry







