In the VII Symposium «Nuclear Magnetic Resonance in Chemistry, Physics and Biological Sciences» that took place on 25-28 of September 2012 in Warsaw, Poland, we presented a talk:
Peter Tolstoy, Benjamin Koeppe, Jing Guo, Svetlana Pylaeva, Elena Tupikina, Gleb Denisov, Hans-Heinrich Limbach
«Effects of Solvation Shell Structure and Dynamics on Geometry and NMR/UV-vis Spectra of H-bonded Complexes»
and two posters:
1) Elena Tupikina, Peter Tolstoy, Sergey Smirnov, Nikolai Golubev, Gleb Denisov «Hydroxyphenyl Benzimidazole as Catalyst for Hydrolysis Reaction: Serine Protease Active Site Model»
2) Svetlana Pylaeva, Peter Tolstoy, Benjamin Koeppe, Christoph Allolio, Daniel Sebastiani, Gleb Denisov «Distribution of Hydrogen Bond Geometries and Spectroscopic Parameters Due to the Solvent-Solute Interactions Studied by Means of Computer Simulations».
«Effects of Solvation Shell Structure and Dynamics on Geometry and NMR/UV-vis Spectra of H-bonded Complexes»
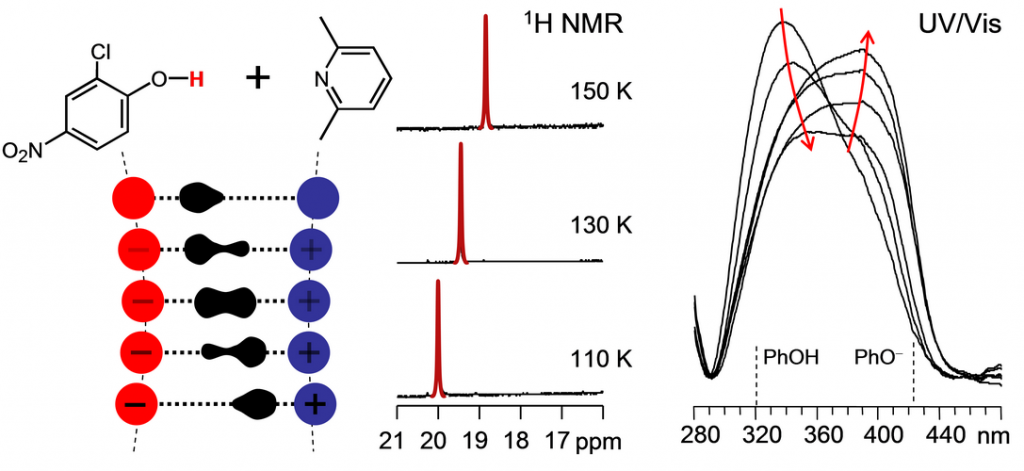
Geometries of strong H-bonded complexes in solutions are quite susceptible to steric hindrances, local electric fields and weak solvent-solute interactions. Thermal fluctuations within the complex as well as fluctuations of the solvent molecules and couterions (for charged systems) in the solvation shell lead to a constant re-arrangement of the H-bond geometry. As a result, a distribution of rapidly interconverting solvent configurations – “solvatomers” – is created, with the lifetime usually not exceeding 10-9 s. This process is fast on the NMR time scale and NMR signals of solvatomers are averaged out into a single narrow peak. In contrast, the time scale of optical spectroscopy is much shorter and spectral bands of individual isomers can be observed individually or as parts of the inhomogeneously broadened band.
In this work we summarize the experimental results obtained over the last couple of years for neutral (OHN) and anionic (OHO-) strong H-bonded complexes dissolved in polar aprotic solvents (CDF3/CDF2Cl, CDCl3, CD2Cl2) by means of low-temperature NMR and combined NMR/UV-Vis spectroscopy. Main types of interacting partners include carboxylic acids, phenols any pyridines. Using so called “hydrogen bond correlations” we are able to interpret spectral features in terms of interatomic distances.
Analyzing the H-bond geometries we reconstruct an “adiabatic” proton transfer pathway and attempt to identify the essential solvent-solute interactions which could explain the experimentally observed trends. Example in the Figure shows1H NMR and UV-vis data for a 2-chloro-4-nitrophenol complex with 2,6-lutidine as well as resulting qualitative pathway for the proton transfer. The roles of solvent-solute dipole-dipole interactions, solvent ordering around the localized charges, counterion effects and weak H-bonding with the acidic CH groups of organic solvents are discussed.



