- Details
- Hits: 1817
V.V. Sokolov, A.Yu. Ivanov, M.S. Avdontseva, A.A. Zolotarev
«Stereochemistry and NMR Spectra of Some Tricyclic Condensed Thiazolidine Derivatives with a Bridgehead Nitrogen Atom»
Chem. Hetercycl. Compd. 2014, 50, 550-556
DOI: 10.1007/s10593-014-1506-3

The configuration of a series of tricyclic condensed thiazolidines with a bridgehead nitrogen atom, for which erroneous data had been published, was determined by X-ray structural analysis and NMR spectroscopy.
- Details
- Hits: 2008
V.A. Rassadin, E. Nicolas, Y. Six
«Ti(OiPr)4/nBuLi: an attractive reagent system for [2+2+2] cyclotrimerisation reactions»
Chem. Commun. 2014, Advance Article
DOI: 10.1039/C4CC02698E

A convenient method for the [2+2+2] cyclotrimerisation of alkynes using Ti(OiPr)4/nBuLi is presented. Homotrimerisation of arylacetylenes proceeds within minutes with excellent regioselectivity. Moreover, the intermolecular construction of ABB heterotrimers can be achieved selectively from two different alkynes with similar electronic properties. The method is also suitable for the synthesis of pyridines.
- Details
- Hits: 2106
J. Malinina, T.Q. Tran, A.V. Stepakov, V.V. Gurzhiy, G.L. Starova, R.R. Kostikov, A.P. Molchanov
«[3+2] Cycloaddition reactions of arylallenes with C-(N-arylcarbamoyl)- and C,C-bis(methoxycarbonyl)nitrones and subsequent rearrangements»
Tetrahedron Lett. 2014, 55, 3663-3666
DOI: 10.1016/j.tetlet.2014.04.107
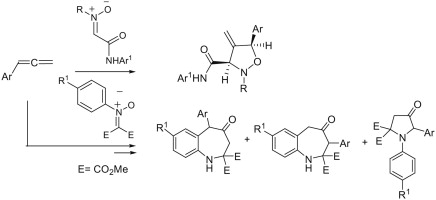
The first example of 1,3-dipolar cycloaddition reactions of nitrones with arylallenes is described. C-Carbamoylnitrones react with the C1single bondC2 double bond of arylallenes affording the corresponding 4-methyleneisoxazolidines in good yields. N-Aryl-C,C-bis(methoxycarbonyl)nitrones usually gave rearranged products: mixtures of 4,5-dihydro-4-oxo-1H-benzo[b]azepine-2,2(3H)dicarboxylates and 4-oxo-1,5-diarylpyrrolidine-2,2-dicarboxylates.
- Details
- Hits: 2047
The second part of the G-RISC workshop “NMR as interdisciplinary tool for the study of non-covalent interactions” took place on 16 and 18 June.
The program was as follows:
• Regina Islamova “Transition Metal-Catalyzed polymerisation: NMR and EPR study”;
• Roman Yakovenko “NMR study of protonation of carbonyl compounds (fluorinated enones and furanes) in Brønsted superacids”;
• Konstantin Luzyanin “Combining NMR and MS tools for study of nano clusters in solution”;
• Kirill Kulish “Zinc(II)-Mediated Generation of 5-amino-1,2,4-oxadiazoles from amidoximes and cyan amides”
- Details
- Hits: 2018
P. R. Golubev, A. S. Pankova, M. A. Kuznetsov
“Transition-Metal-Free Approach to 4-Ethynylpyrimidines via Alkenynones”
Eur. J. Org. Chem. 2014, 3614-3621
DOI: 10.1002/ejoc.201402045

A practical approach to the synthesis of 4-ethynylpyrimidines by the condensation of arylamidines with 2-aryl-1-ethoxy-5-(trimethylsilyl)pent-1-en-4-yn-3-ones has been developed. As these latter ketones are easily accessible from bis(trimethylsilyl)acetylene and arylacetyl chlorides, the regioselective condensation reported herein provides a facile access to both TMS-protected and unprotected 4-ethynylpyrimidines in yields of up to 85%.







