В. Хуанby W. Huang, C. Li, L. Zeng, J. Zhang, M. Kurochkin, I. Kolesnikov, Z. Umar, J. Zhang, W. Liu, Á. Kukovecz, M. Kurboniyon and X. Zhang
Inorg. Chem. 2025, 64, 24, 12100–12111
https://doi.org/10.1021/acs.inorgchem.5c01428
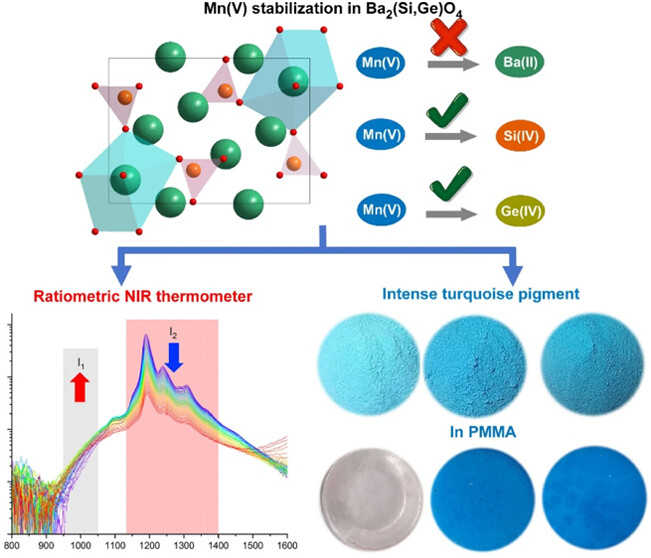 Luminescence of transition metal ions in unusual oxidation states is a treasure for developing new phosphors. Pentavalent manganese (Mn5+), which possesses a 3d2 electron configuration, has been reported less compared to its Mn2+ and Mn4+ counterparts due to the difficulty in its stabilization. In this study, Ba2(Si1–xGex)O4:Mn5+ phosphors were synthesized by a solid-state reaction method. The successful Mn5+ stabilization in the (Si,Ge)O4 tetrahedron leads to a bright turquoise body color with a strong NIR-II sharp-peak emission at room temperature. By replacing Si4+ with Ge4+, the peak position of the 1E–3A2 transition remains at 1181 nm, while the corresponding integrated intensity is enhanced by 150% at x = 0.6. First-principles density functional theory (DFT) calculations were performed to explore the geometrically optimized structure and electronic structure variation of the Ba2(Si,Ge)O4:Mn5+ phosphor. Then, they are discussed in association with the observed luminescence properties. It is found that modification of the host composition could modify the accelerated thermal quenching rate of the 1E emission. Temperature can be read from Mn5+1E vs 3T2 luminescence intensity ratios. This work represents a step toward exploring the unusual Mn5+ as the emitting center for the next-generation NIR-II phosphors and chromophores for new pigments.
Luminescence of transition metal ions in unusual oxidation states is a treasure for developing new phosphors. Pentavalent manganese (Mn5+), which possesses a 3d2 electron configuration, has been reported less compared to its Mn2+ and Mn4+ counterparts due to the difficulty in its stabilization. In this study, Ba2(Si1–xGex)O4:Mn5+ phosphors were synthesized by a solid-state reaction method. The successful Mn5+ stabilization in the (Si,Ge)O4 tetrahedron leads to a bright turquoise body color with a strong NIR-II sharp-peak emission at room temperature. By replacing Si4+ with Ge4+, the peak position of the 1E–3A2 transition remains at 1181 nm, while the corresponding integrated intensity is enhanced by 150% at x = 0.6. First-principles density functional theory (DFT) calculations were performed to explore the geometrically optimized structure and electronic structure variation of the Ba2(Si,Ge)O4:Mn5+ phosphor. Then, they are discussed in association with the observed luminescence properties. It is found that modification of the host composition could modify the accelerated thermal quenching rate of the 1E emission. Temperature can be read from Mn5+1E vs 3T2 luminescence intensity ratios. This work represents a step toward exploring the unusual Mn5+ as the emitting center for the next-generation NIR-II phosphors and chromophores for new pigments.



