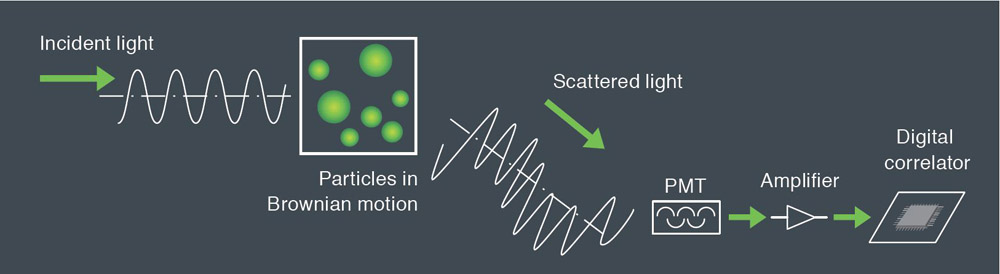
Dynamic light scattering is the measurement of fluctuations in scattered light intensity with time. These fluctuations in intensity arise due to the random Brownian motion of the nanoparticles. Therefore, the statistical behavior of these fluctuations in scattered intensity can be related to the diffusion of the particles. Since larger particles diffuse more slowly than small particles one can readily relate particle size to the measured fluctuations in light scattering intensity. With modern instruments such as the SZ-100 the technique is rapid and reliable.
Absorption spectroscopy is used to obtain the absorption spectra (transmission), diffuse scattering and measurement of the optical density of matter in different states of aggregation in the ultraviolet, visible and near-infrared regions of the spectrum. This method is used to the vast majority of information about the structure of the material at the atomic and molecular level, how atoms and molecules behave when combined in condensed matter. Feature of optical spectroscopy compared to other types of spectroscopy is most structurally organized matter (larger atoms) interacts resonantly with the electromagnetic field in an optical frequency range. Therefore, optical spectroscopy is widely used for studying matter.
ОFluorescent spectroscopy differ from other spectroscopic techniques because of the recorded spectral dependence is a function of two variables - the excitation wavelength λex and emission wavelength λem. If λex is kept constant and λem is scanned, the measured luminescence spectrum is emission (spectral dependence of the luminescent intensity on the wavelength). If you are scanning λex at constant λem, you measure excitation spectrum (spectral dependence of the excitation efficiency on the excitation wavelength).
FT-IR spectroscopy (FT-IR) is a well-known and proven technology for analysis and identification of unknown chemicals. The method is based on a microscopic interaction between infrared light and a chemical substance by a process of absorption and results in a set of ranges, called the spectrum (the spectrum is unique for chemical substance and serves as a "molecular fingerprint"). Despite the fact that FT-IR - is widely applicable method, it uses the analysis of the chemical substances’ intrinsic properties, thanks to that FT-IR is very suitable for comparison with the spectral library. With the use of an extensive database, the comparison with the spectral library makes it possible to quickly identify thousands of chemicals on the base of their unique "molecular fingerprint".
The Raman scattering - non-contact non-destructive method for analyzing the structure of substances. The basic idea of the Raman spectroscopy method is the registration of inelastically scattered light from the sample with subsequent decoding of the spectrum obtained by comparing the frequencies of the oscillations with the characteristic oscillations that is unique to each substance. Using such analysis, it is possible to determine the symmetry of the sample, the frequency of the vibrational modes and quality of the grown structures as well as the content of alloying and undesirable impurities, the distribution of the elastic deformation, etc. Here are just some applications that can be solved with the help of Raman spectroscopy:
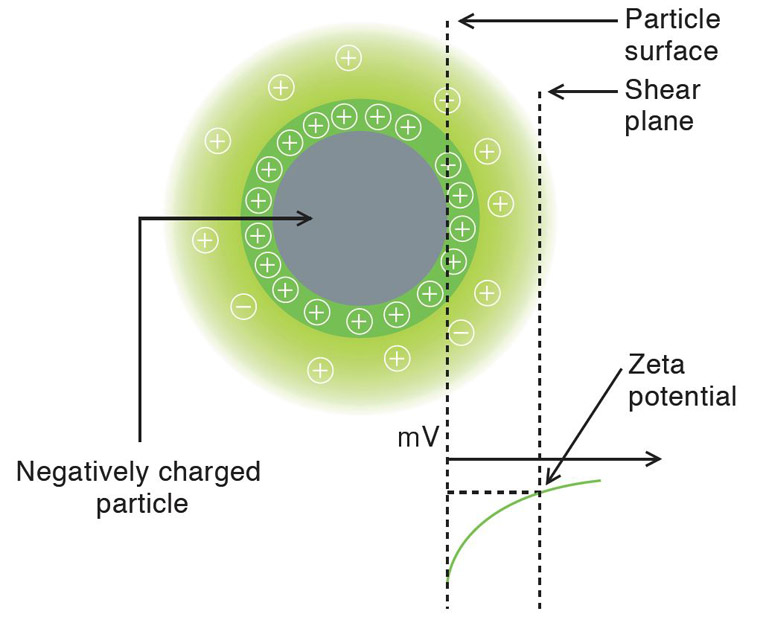
Many nanoparticles or colloidal particles have a surface charge when they are suspension. When an electric field is applied, the particles move due to the interaction between the charged particle and applied field. The direction and velocity of the motion is a function of particle charge, the suspending medium, and the electric field strength. Particle velocity is then measured by observing the Doppler shift in the scattered light. The particle velocity is proportional to the electrical potential of the particle at the shear plane which is zeta potential. Thus, this optical measurement of the particle motion under an applied field can be used to the determine zeta potential.
Inorganica Chimica Acta Volume 455, Part 1, 30 January 2017, Pages 9–14
Dmitrii S. Bolotin, Mikhail V. Il’in, Ilya E. Kolesnikov, Vitaliy V. Suslonov, Yuri Novozhilov, Oksana Ronzhina, Mikhail Krasavin, Vadim P. Boyarskiy, Renier Koen, Andreas Roodt
Fluorescent (pyrazolyl acetoxime)ZnII complexes: synthetic, structural, and photophysical studies
Inorganica Chimica Acta Volume 455, Part 1, 30 January 2017, Pages 9–14
DOI: 10.1016/j.ica.2016.10.008
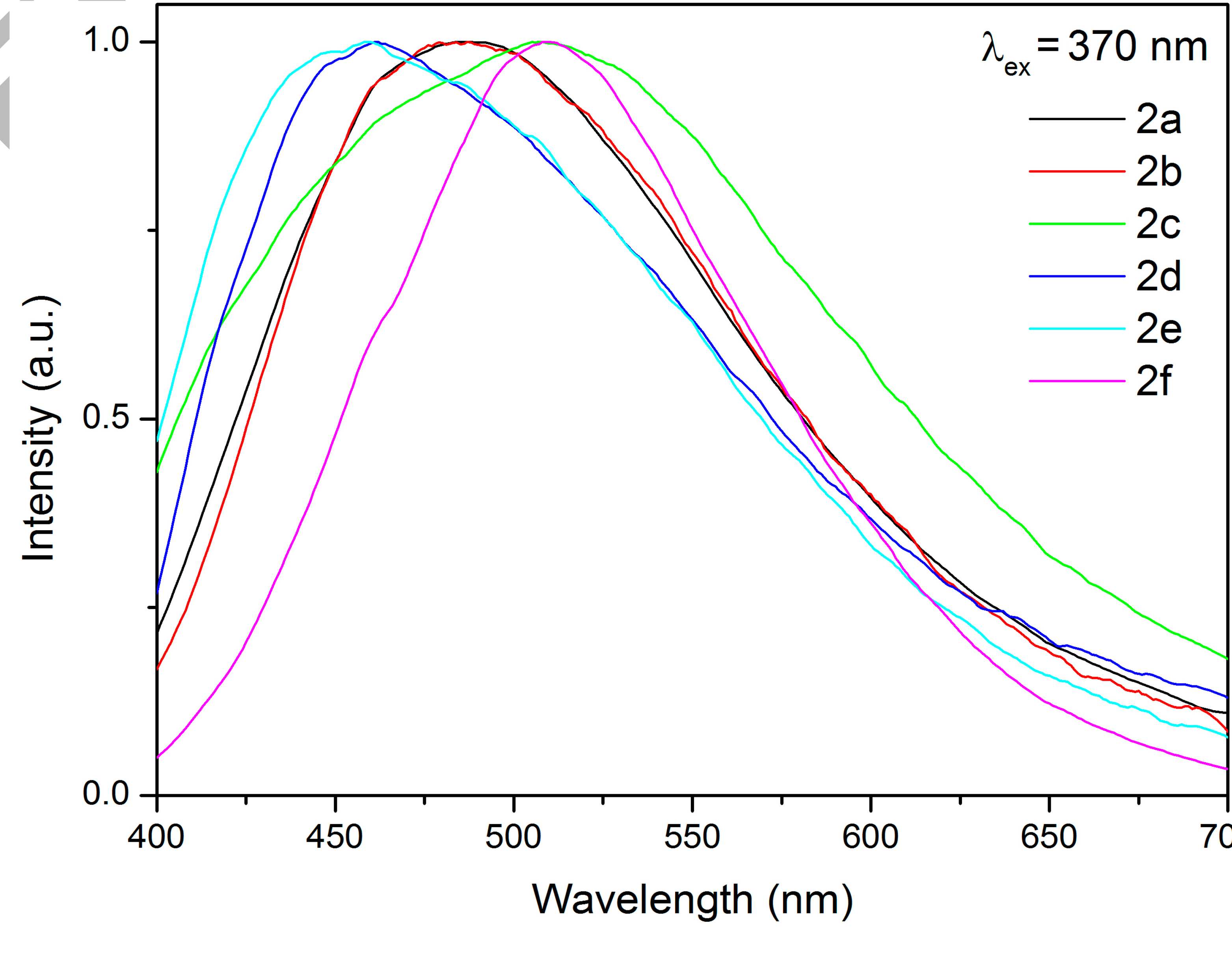
Treatment of pyrazolyl acetoximes p-RC6H4{CN(H)NCCH}CH2C(Me)=NOH (R = OMe, H, F, Cl, CN, NO2) with 1 equiv of ZnCl2 leads to the complexes [ZnCl2(p-RC6H4{CN(H)NCCH}CH2C(Me)=NOH)] (81–91%). These species exhibit fluorescence at room temperature with emission maxima in the region 460–509 nm and biexponential relaxation with τ1 = 4.63–8.64 ns and τ2 = 0.482–0.955 ns, respectively. The zinc complexes were characterized by elemental analyses (C, H, N), HRESI-MS, FTIR, 1H and 13C{1H} NMR, and CP-MAS TOSS 13C{1H} NMR. In addition, two zinc complexes and one pyrazolyl acetoxime were studied by single-crystal X-ray diffraction.
Journal of Molecular Liquids Volume 230, March 2017, Pages 113–120
V.S. Fundamensky, T.A. Kochina, Y.A. Kondratenko, A.A. Zolotarev, Yu.G. Vlasov, I.S. Ignatyev
Ionic liquids based on triethanolammonium salts of dicarboxylic acids (oxalic, malonic, succinic). Crystal structure and cation-anion interaction
Journal of Molecular Liquids Volume 230, March 2017, Pages 113–120
DOI: 10.1016/j.molliq.2016.12.111
The series of ionic liquids based on triethanolammonium salts of dicarboxylic acids (oxalic, malonic and succinic) was synthesized, characterized by IR and H1, C13 NMR spectroscopy, thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). Their structure was determined by single-crystal X-ray diffraction. It was found that triethanolammonium [(HOCH2CH2)3NH]+ cations in salts of oxalic (1), malonic (2) and succinic acids (3) containing monoanions [OOC(CH2)nCOOH]- (n=0-2) have the endo conformation and the ammonium proton (Ham) resides inside the “lampshade” formed by three CH2CH2OH branches connected with nitrogen atom. The asymmetric cell of the second modification of the succinic acid salt (4) includes two triethanolammonium cations and a succinate dianion [(HOCH2CH2)3NH]2+[OOC(CH2)2COO]2-. In this salt (4) one CH2CH2OH branch of the triethanolammonium cation is rotated around the N-C bond (endo-exo conformation) and forms infinite TEA chains. This pattern was firstly found in organic salts of TEA. The obtained results show that different structures of triethanolammonium cations in the salts of dicarboxylic acids have significant influence on the cation-anion interaction.



