by A. Kishkentayeva, K. Kopbalina, Z. Shaimerdenova, E. Shults, Y. Gatilov, D. Pankin, M. Smirnov, A. Povolotckaia, D. Turdybekov and N. Mazhenov
Materials 2025, 18(13), 3153;
https://doi.org/10.3390/ma18133153
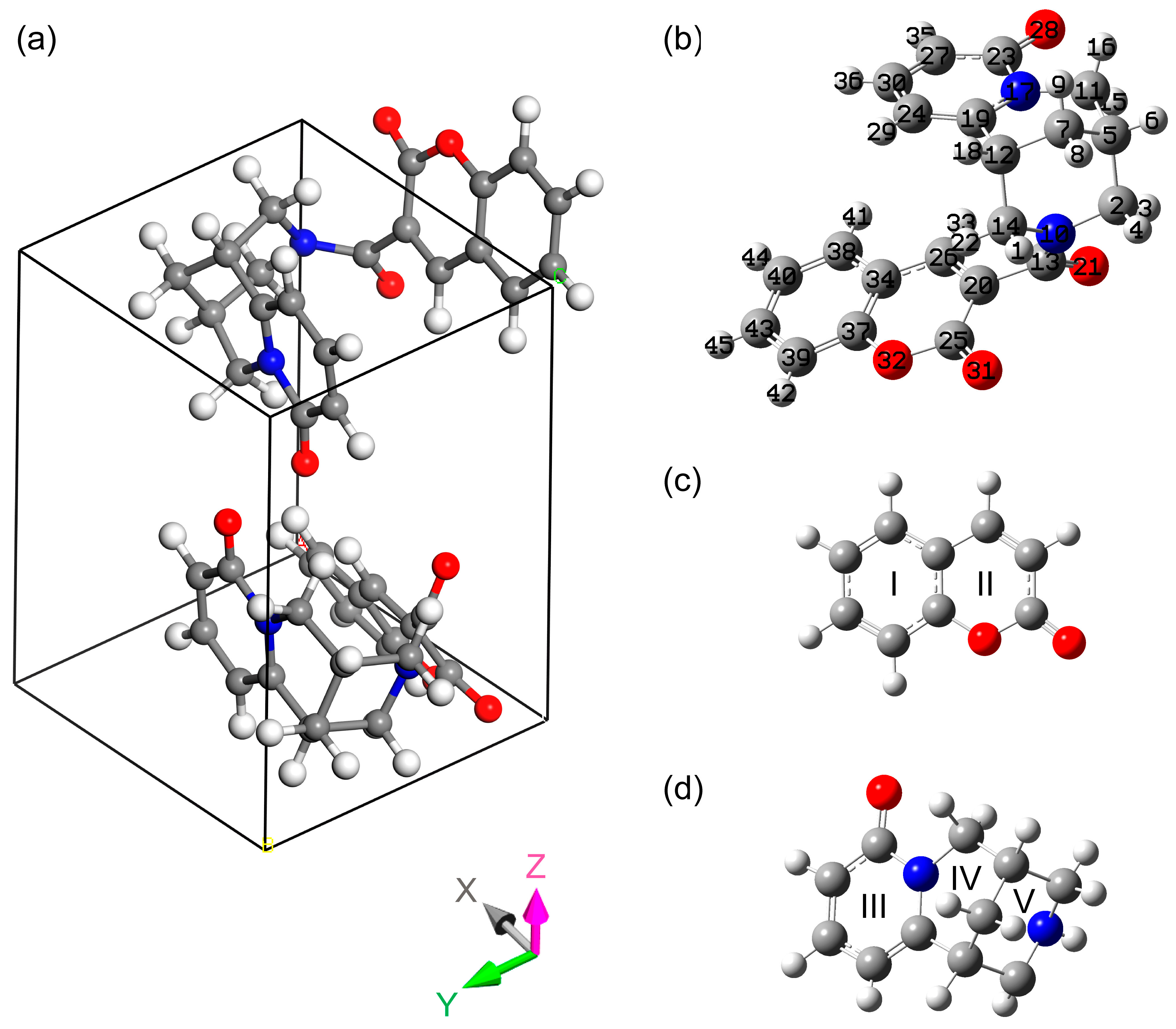
Coumarin and cytisine and their derivatives have significant biological activity. In addition, the electronic properties of coumarin derivatives are very sensitive to the molecular environment, which allows for their use as sensors for bioluminescent imaging. Due to the fact that cytisine exhibits high activity in binding to nicotinic acetylcholine receptors, a compound combining parts of cytisine and coumarin may have a broader spectrum of biological activity and also act as a photoactive element for promising use in optoelectronic devices. This article reports the synthesis of a crystalline cytisine–coumarin complex (IUPAC: N-(2-oxo-2H-chromene-3-carbonyl)cytisine), along with the results of both theoretical and experimental investigations of its structural and electronic properties. The structure of this new compound was established on the basis of X-ray diffraction and Fourier transform infrared spectroscopy data and was confirmed through density functional theory calculations using periodic crystal and single-molecule approaches. Interpretations of the IR absorption peaks and the atomic patterns of the vibrational modes are given. The electronic band structure and the contributions of individual atoms to the electronic density of states are analyzed. The structural and optical properties considered may be useful for quality control of the compound and for studying similar matrices.



