Brief description of the method
NMR in zero field is used to study magnetically ordered compounds. The method is sensitive to changes in crystal structure and magnetic structure of the material, to the local magnetic fields and magnetic moment of atoms.
NMR in zero field can be used to study crystals, amorphous materials (ferromagnetic metastable alloys, amorphous semiconductors etc.), some liquids (for example, ferromagnetic liquids) and gases inside solid materials. Ferromagnetics, antiferromagnetics, ferrimagnetics and some materials which are not magnetically ordered but have in their structure magnetically ordered compounds inducing local magnetic fields on neighboring atoms and ions.
Interpretation of 1D and 2D NMR spectra in zero field gives information about the number of crystalline phases, presence of defects, types of defects, number of non-equivalent local environments of a nucleus etc.
Examples of studied systems:
Among chemical elements ferromagnetism is displayed by Fe, Со and Ni (3d-metals) and rare earth metals Gd, Tb, Dy, Ho, Er.
Among compounds ferromagnetism is displayed by many bimetallic and multi-metallic alloys as well as their combinations with various non-ferromagnetics, alloys and compounds containing Cr and Mn with non-ferromagnetics (Heusler alloys), ZrZn2 and ZrxM1-xZn2 (where М = Ti, Y, Nb or Hf), Au4V, Sc3In etc., some chemicals containing actinoids (f.e. UH3).
Among chemical elements antiferromagnetism is displayed by solid oxygen, Cr, as well as a number of rare earth metals (Dy, Ho, Er, Tm, Tb).
The number of antiferromagnetic compounds is huge. For example: MnSO4, FeSO4, CoSO4, NiSO4, MnCO3, FeCO3, MnO, FeF2 etc.
Ferrimagnetism is displayed by ionic crystals containing magnetic ions of various elements, ferrites, fluorides (f.e. RbNiF3, CsNiF3, TlNiF3, CsFeF3), some alloys and intermetallic compounds. In most cases these are compounds containing rare earth elements.
Additional materials
1. Spin echo review: http://www.fis.unipr.it/dokuwiki/doku.php?id=andrey.sidorenko:nmr
2. Applications of NMR in zero field:
• http://wrap.warwick.ac.uk/28083/
• http://iopscience.iop.org/0953-8984/24/15/156001/article
• http://www.jastmag.org/journal/view.php?Type=C&number=62
• http://nmr.mff.cuni.cz/downloads/Gamaliy_Abstract.pdf
1. 1D spectra
1D NMR spectra in zero filed give information about the phase composition of the sample, about the presence of certain crystalline structures, defects, about the number of non-equivalent positions in which the studied nuclei are located, about the magnetic structure of the material.
Example:
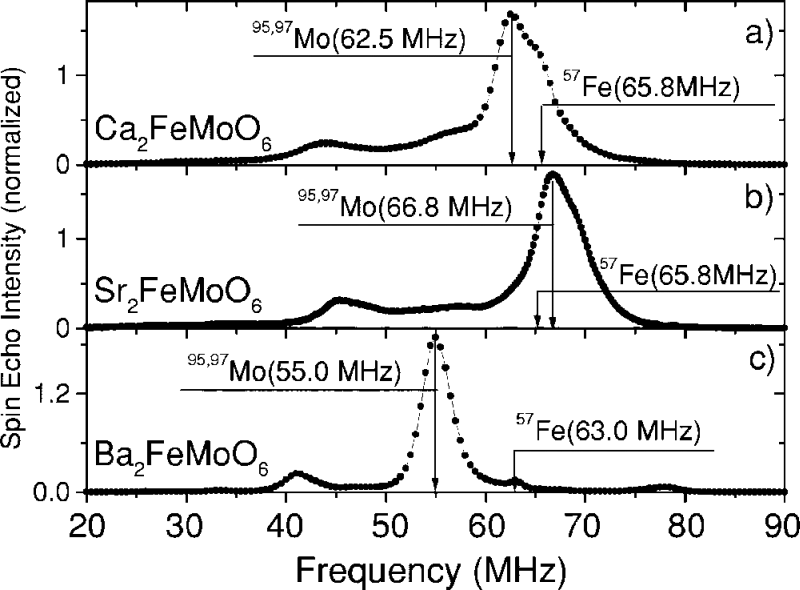
NMR spectra in zero field of compounds Ca2FeMoO6, Sr2FeMoO6, and Ba2FeMoO6; T= 4.2 K.
Adapted from M. Wojcik, E. Jedryka, S. Nadolski, Phys. Rev. B, 2005, 71, 104410.
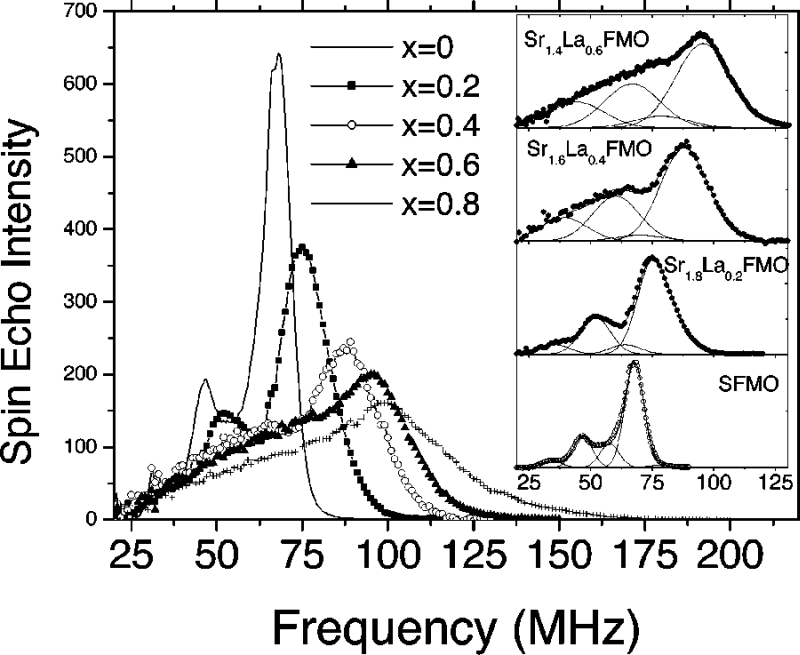
NMR spectra of Sr2-xLaxFeMoO6. T = 4.2 K.
Adapted from Wojcik, E. Jedryka, S. Nadolski, Phys. Rev. B, 2004, 69, 100407.
2. 2D spectra
2D NMR spectra of magnetically ordered compounds combine the system response to the changes of frequency and amplitude of the pulse.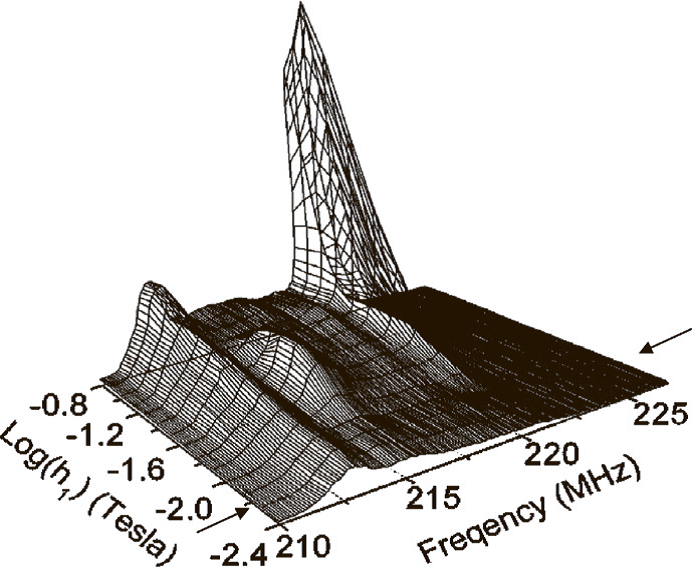
2D NMR spectrum is zero field of powdered cobalt at 300 K. Adapted from R. Speight, A.Wong, P.Ellis, T.Hyde, P.T.Bishop, M.E.Smith, Solid State NMR, 2009, 35, 67-73.
3. Spin-lattice relaxation time Т1
The spin-lattice relaxation time T1 characterizes the rate with which the system of spins reaches the thermal equilibrium with the other degrees of freedom. During the relaxation the population difference between the spin energy level creates the macroscopic magnetization of the sample; in other words, T1 is the characteristic time needed for the magnetization of the sample.
Several methods can be used for T1 measurements:
- pulse sequence 90°—τ—90° (if T1≈ T2 ≈ T2*).
- pulse sequence 180°—τ—90° (“inversion-recovery”) or stimulated echo (if case of short T2*).
- saturation method.
4. Spin-spin relaxation time Т2
T2 characterizes the time needed for the recovery of the transverse magnetization to its equilibrium (zero) value. For the measurement of T2 time the pulse sequence 90°—τ—180° is used.



