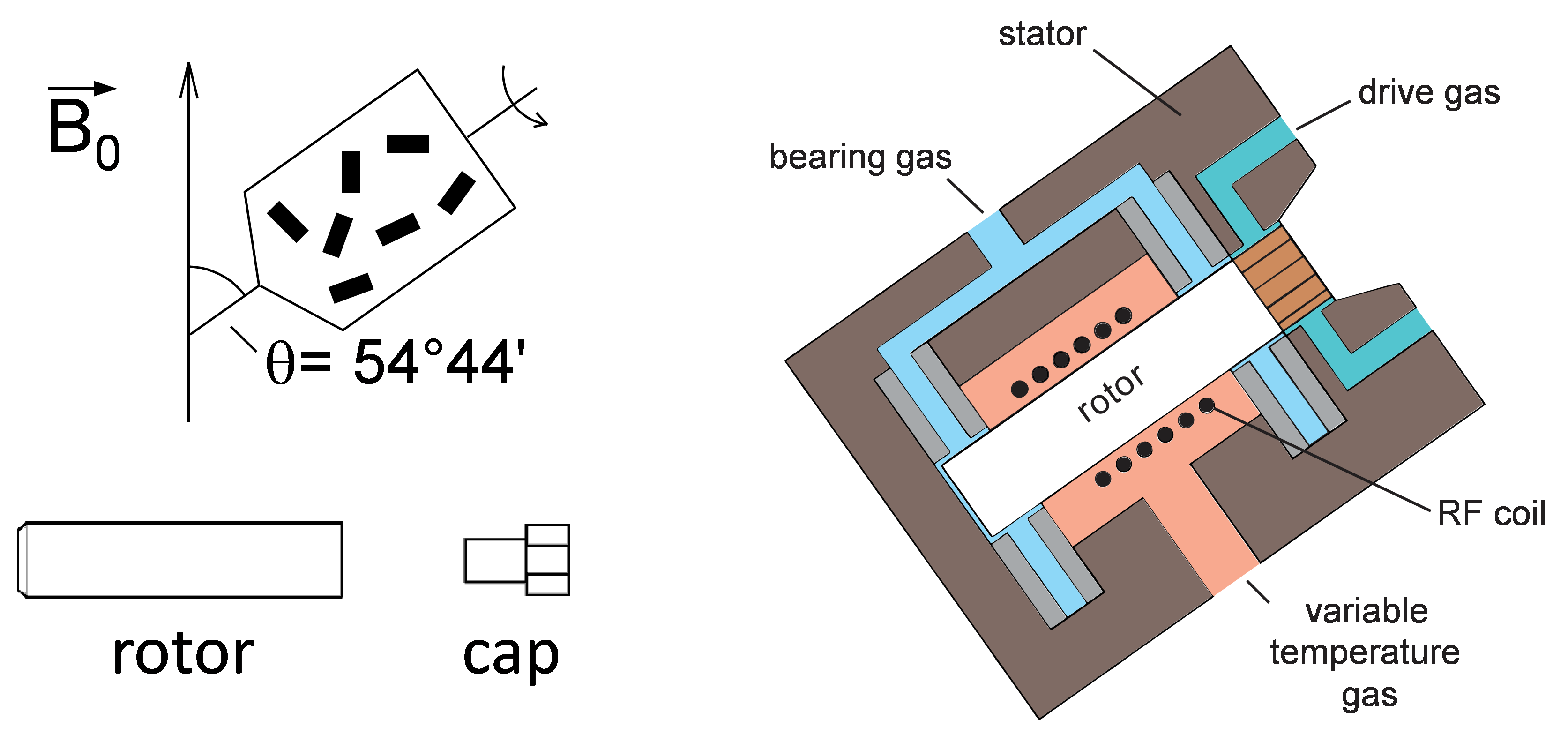
Fig. 1. Schematic representation of MAS rotor and stator.
In order to spin the sample, it should be packed in special rotor which is usually made of zirconia and closed tightly with a cap (Fig. 1, right). Rotors have different diameters that vary from 1.3 to 7 mm in modern spectrometers. The smaller the diameter, the higher MAS speed is achievable. The typical volume for regular 4 mm rotor is 80 μL. In special cases or when there is not enough sample available, reduced volume rotors with different inserts are used which allow to pack 50 or even 12 μL of sample being certain that it is located in the center of the RF coil. That is essential the sample to be packed so that the spinner is well balanced, because an unbalanced spinner can crash suddenly, causing severe damage to the coil, stator and other probehead parts.
In the case when spinning rate is less than the magnitude of the anisotropic interaction, additional artifactual lines appear in the spectrum which are separated by the rate of spinning in Hz and are called spinning sidebands (SSB) (Fig. 2). In publications they usually are marked with asterisks to distinguish them from the “real” lines. There exist a number of methods to get rid of these artifacts (Fig. 3), though in some studies they are useful or even essential. Cross polarization is often used in combination with the magic angle spinning (CP MAS, see the corresponding section).

Fig. 2. 13C NMR spectra of glycine at different MAS speeds. Even at 12 kHz MAS rate small SSB are still present. Note that isotropic line is not necessarily the most intensive one and its chemical shift doesn’t depend on the spinning rate.

Fig. 3. 13C NMR spectra of glycine (MAS 4.5 kHz): normal CP and CP TOSS (Total Sideband Supression).
2. Cross Polarization (CP)
The technique allows one to transfer the magnetization from the highly polarized nuclei (usually 1H) to the neighboring weakly polarized nuclei (such as 15N or 29Si). In most cases this allows one to significantly shorten the experiment time, but at the cost of partially losing the information about the integral intensities of the signals.
In the case of heavy nuclei they not only often exhibit low natural abundance of isotopes with spin quantum number 1/2, such as 29Si, 15N and 13C, but also have small gyromagnetic ratio compared to that of the proton, which leads to small Zeeman splitting of the energy levels. Thus the signal-to-noise ratio in their spectra is often far from satisfactory. Moreover, the spin-lattice (T1) relaxation time in solids can be very long, reaching dozens of minutes and even longer.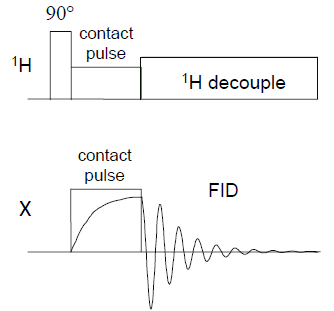
In order to enhance the signals from rare nuclei a Cross Polarization (CP) pulse sequence is used (see the figure). It utilizes the effect of “flow” of the magnetization from highly polarized nuclei (most commonly, 1H) to lower polarized ones via the dipolar coupling between them when they are brought into contact, which results in more intensive NMR signal and better signal-to-noise ratio.
Another advantage of CP technique is that the sampling rate depends on the relaxation time of nuclei from which the magnetization is transferred, which helps in case of 1H nuclei, because they typically relax much faster than other 1/2 spin nuclei, allowing more scans to be acquired over the same time, as compared to simple single-pulse experiment that detects rare nuclei directly.
3. CPPI, CPPISPI and CPPIRCP
These spectral editing methods help to distinguish signals from CH3, CH2, CH and quaternary carbon atoms.
CPPI, CPPISPI are CPPIRCP are spectral editing pulse sequences, which allow one to distinguish signals from CH3, CH2, CH and quaternary carbon atoms. For these experiments one needs medium-fast spinning rates (up to 10 kHz)
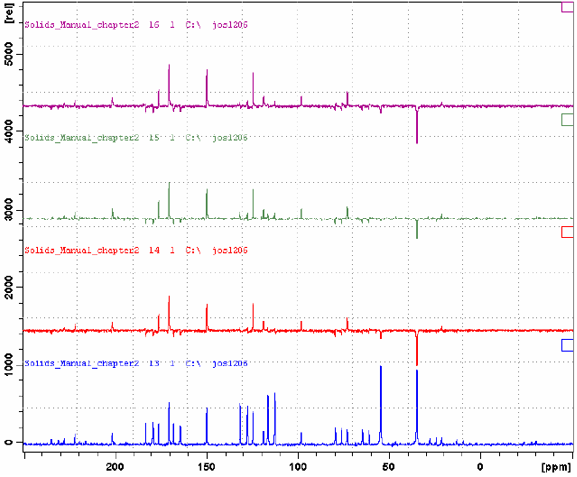
Figure: CP/MAS spectrum of tyrosine hydrochloride (MAS 6.5 kHz); blue — without spectral editing; red – CPPI (CH2 signals have negative intensity, aromatic CH signals are suppressed, CHa signal is weak and negative); green – CPPIRCP (CH are better suppressed, though aromatic signals are somewhat stronger); violet – CPPISPI. (for more details see Bruker Avance Solids Manual)
4. Double Cross Polarization (DCP)
Two (or more) consequitive CP experiments
Sometimes for the structure determination the double cross polarization technique might be useful. In this experiment two (or more) consecutive CP experiments are being applied: the magnetization is transferred from the first type of nuclei to the second one and after that from the second type to the third one. In the example below the results of the 1H → 15N → 13C DCP experiment on histidine are shown. Top spectrum: 13C CPMAS NMR (Bruker 400 МГц, WB, rotor 4 mm, MAS 11 kHz). Bottom spectrum: 13C DCPMAS 1H → 15N → 13C (the signals are visible only for those carbon atoms which are in dipole-dipole interaction with nitrogen atoms).
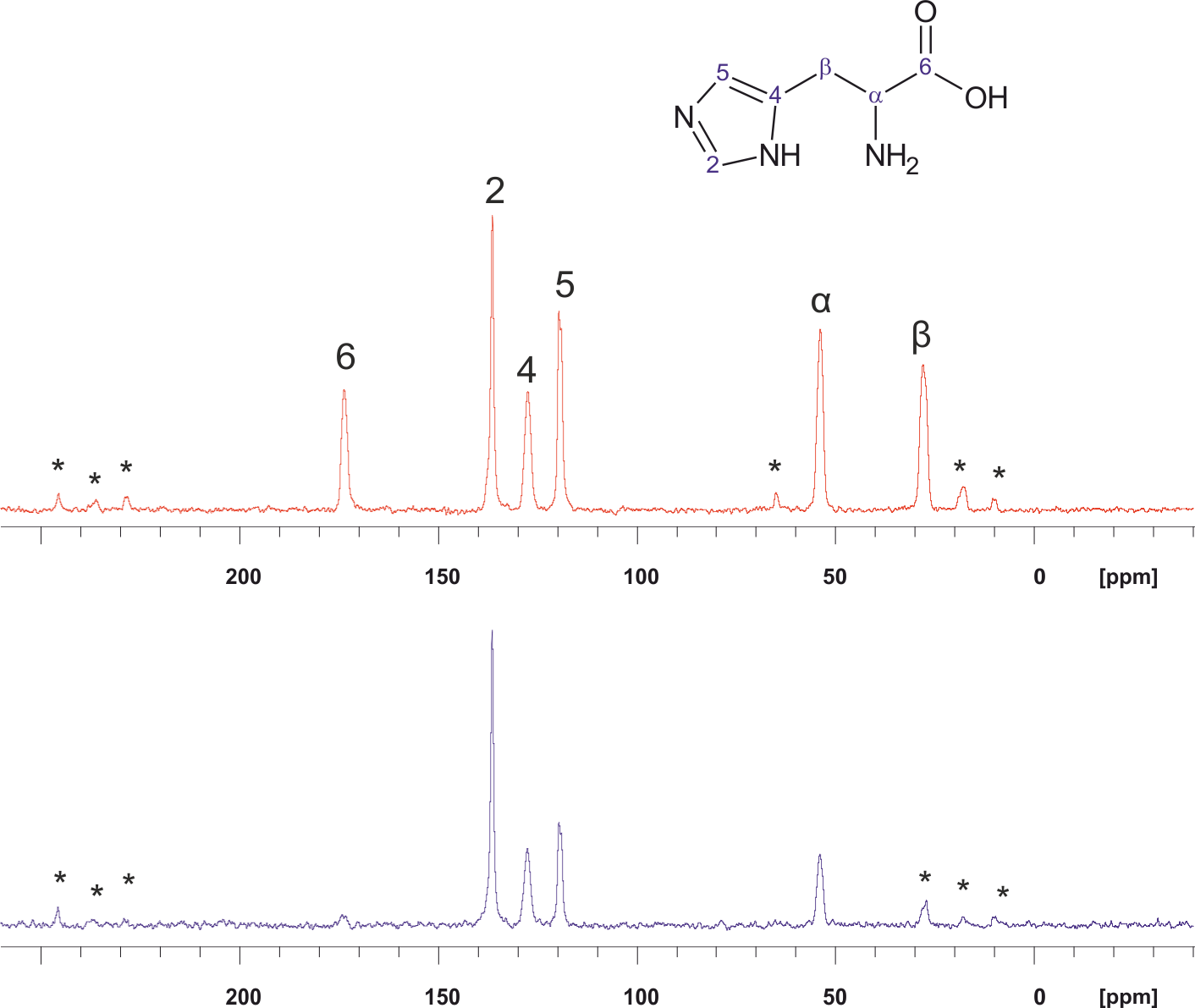
* — rotational sidebands
5. Homonuclear Decoupling
This method is used for the simplification of complex multiplets in spectra of highly abundant nuclei, such as 1H, 19F and 31P.
Homonuclear decoupling is used for the simplification of complex multiplets in spectra of highly abundant nuclei, such as 1H, 19F and 31P. In the simplest case of an AX spin system its spectrum consist of the doublet at νA frequency and a doublet at νX frequency. Continuous low-power irradiation at νA removes the scalar interaction and the spectrum is simplified to the singlet at νХ frequency. In case of a more complicated spin systems the spectrum could also be partially simplified in a similar fashion.

6. Heteronuclear Correlation (HETCOR)
Correlation between 1H NMR chemical shifts and X NMR chemical shifts.
FSLG HETCOR experiment correlates 1H NMR chemical shifts with chemical shifts of X-nuclei (such as 13С or 15N). The experiment gives excellent resolution in indirect 1H dimension. The homonuclear decoupling during the 1H evolution period is achieved by using the FSLG (Frequency Switched Lee Goldburg) pulse sequence, which works even at higher MAS rates. The proton decoupling is not strictly necessary because high MAS rates already give that.
The mixing occurs during the contact pulse for magnetization transfer. As magnetization from protons to X-nuclei happens rather fast, the contact time should be short in order to prevent magnetization transfer for larger distances (creation of non-specific cross-peak pattern). Modification of the basic pulse sequence exist, which utilize the magnetization transfer at the LG shift frequency for protons. In the latter case the magnetization is being transferred to X nucleus from the nearest protons only.
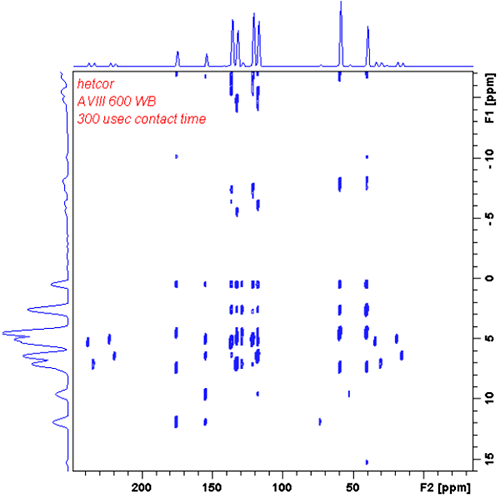
FSLG HETCOR spectrum of tyrosine hydrochloride (from Bruker Avance Solids Manual)
7. Homonuclear Recoupling

13C CP (top) and PostC7 (bottom) homonuclear recoupling NMR spectra of tyrosine hydrochloride recorded on Bruker 400 MHz, WB, rotor 4 mm, MAS 10 kHz. Theoretically maximal efficiency of double quantum transfer is 70%.
8. Radio Frequency-Driven Recoupling (RFDR)
Correlation of chemical shifts under MAS; allows one to estimate interatomic distances.
RFDR with longitudinal magnetization transfer is a homonuclear dipole-dipole recoupling experiment, which allows one to correlate chemical shifts under MAS conditions. The dependence of cross-peak intensities on time might be used to estimate the interatomic distances. At short times of dipole-dipole recoupling the cross-peak arise only from those spins, which are in close proximity of each other.
Homonuclear dipole-dipole recoupling in this experiment is achieved by using the 180-degree pulse synchronized with the frequency of the rotor rotation (one pulse per cycle).

2D RFDR spectrum of fully-13C-labelled histidine (from Bruker Avance Solids Manual).
9. Double Quantum – Single Quantum Correlation

10. Refocused CP INADEQUATE
The method allows one to obtain information about the structure of the carbon skeleton of the molecule based of the values of spin-spin coupling constant between neighboring carbon atoms. Besides the existence of a cross polarization section the pulse sequence for this method is identical to the pulse sequence of liquid-state INADEQUATE. The 13C-13C dipole-dipole interaction is removed by fats MAS conditions. The method requires fine tuning of the decoupling parameters and long acquisition times.

Refocused INADEQUATE 13С-13С spectrum of simvastatin (J. Phys. Chem. A 2004, 108, 3955-3964)



