J. Malinina, T.Q. Tran, A.V. Stepakov, V.V. Gurzhiy, G.L. Starova, R.R. Kostikov, A.P. Molchanov
«[3+2] Cycloaddition reactions of arylallenes with C-(N-arylcarbamoyl)- and C,C-bis(methoxycarbonyl)nitrones and subsequent rearrangements»
Tetrahedron Lett. 2014, 55, 3663-3666
DOI: 10.1016/j.tetlet.2014.04.107
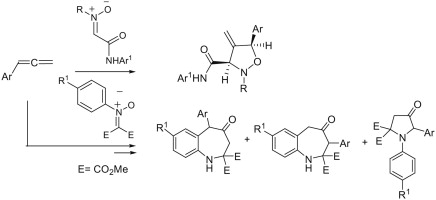
The first example of 1,3-dipolar cycloaddition reactions of nitrones with arylallenes is described. C-Carbamoylnitrones react with the C1single bondC2 double bond of arylallenes affording the corresponding 4-methyleneisoxazolidines in good yields. N-Aryl-C,C-bis(methoxycarbonyl)nitrones usually gave rearranged products: mixtures of 4,5-dihydro-4-oxo-1H-benzo[b]azepine-2,2(3H)dicarboxylates and 4-oxo-1,5-diarylpyrrolidine-2,2-dicarboxylates.




