- Информация о материале
- Просмотров: 1861
Applied Surface Science Volume 416, September 2017, Pages 988-995
A.A. Ionina, S.I. Kudryashova, A.O. Levchenko, L.V. Nguyen, I.N. Saraev, A.A. Rudenko, E.I. Ageev, D.V. Potorochin, V.P. Veiko, E.V. Borisov, D.V. Pankin, D.A. Kirilenko, P.N. Brunkov
Correlated topographic and structural modification on Si surface during multi-shot femtosecond laser exposures: Si nanopolymorphs as potential local structural nanomarkers
Applied Surface Science Volume 416, September 2017, Pages 988-995
DOI: 10.1016/j.apsusc.2017.04.215

High-pressure Si-XII and Si-III nanocrystalline polymorphs, as well as amorphous Si phase, appear consequently during multi-shot femtosecond-laser exposure of crystalline Si wafer surface above its spallation threshold along with permanently developing quasi-regular surface texture (ripples, microcones), residual hydrostatic stresses and subsurface damage, which are characterized by scanning and transmission electron microscopy, as well as by Raman micro-spectroscopy. The consequent yields of these structural Si phases indicate not only their spatially different appearance, but also potentially enable to track nanoscale, transient laser-induced high-pressure, high-temperature physical processes - local variation of ablation mechanism and rate, pressurization/pressure release, melting/resolidification, amorphization, annealing - versus cumulative laser exposure and the related development of the surface topography.
- Информация о материале
- Просмотров: 1742
Optics and Lasers in Engineering Volume 96, September 2017, Pages 63-67
Vadim Veiko, Yulia Karlagina, Mikhail Moskvin, Vladimir Mikhailovskii, Galina Odintsova, Pavel Olshin, Dmitry Pankin, Valery Romanov, Roman Yatsuk
Metal surface coloration by oxide periodic structures formed with nanosecond laser pulses
Optics and Lasers in Engineering Volume 96, September 2017, Pages 63-67
DOI: 10.1016/j.optlaseng.2017.04.014

In this work, we studied a method of laser-induced coloration of metals, where small-scale spatially periodic structures play a key role in the process of color formation. The formation of such structures on a surface of AISI 304 stainless steel was demonstrated for the 1.06 μm fiber laser with nanosecond duration of pulses and random (elliptical) polarization. The color of the surface depends on the period, height and orientation of periodic surface structures. Adjustment of the polarization of the laser radiation or change of laser incidence angle can be used to control the orientation of the structures. The formation of markings that change their color under the different viewing angles becomes possible. The potential application of the method is metal product protection against falsification.
- Информация о материале
- Просмотров: 1529
Optics and Lasers in Engineering 96 (2017) 63–67
Vadim Veiko, Yulia Karlagina, Mikhail Moskvin, Vladimir Mikhailovskii, Galina Odintsova, Pavel Olshin, Dmitry Pankin, Valery Romanov, Roman Yatsuk
«Metal surface coloration by oxide periodic structures formed with nanosecond laser pulses»
Optics and Lasers in Engineering 96 (2017) 63–67
DOI: 10.1016/j.optlaseng.2017.04.014

In this work, we studied a method of laser-induced coloration of metals, where small-scale spatially periodic structures play a key role in the process of color formation. The formation of such structures on a surface of AISI 304 stainless steel was demonstrated for the 1.06 µm fiber laser with nanosecond duration of pulses and random (elliptical) polarization. The color of the surface depends on the period, height and orientation of periodic surface structures. Adjustment of the polarization of the laser radiation or change of laser incidence angle can be used to control the orientation of the structures. The formation of markings that change their color under the different viewing angles becomes possible. The potential application of the method is metal product protection against falsification.
- Информация о материале
- Просмотров: 1445
Zeitschrift für anorganische und allgemeine Chemie Volume 643, Issue 14 August 2017 Pages 895–902
Igor D. Strelnik, Elvira I. Musina, Svetlana N. Ignatieva, Anna S. Balueva, Tatiana P. Gerasimova, Sergey A. Katsyuba, Dmitry B. Krivolapov, Alexey B. Dobrynin, Christoph Bannwarth, Stefan Grimme, Ilya E. Kolesnikov, Andrey A. Karasik, and Oleg G. Sinyashin
Pyridyl Containing 1,5-Diaza-3,7-diphosphacyclooctanes as Bridging Ligands for Dinuclear Copper(I) Complexes
Zeitschrift für anorganische und allgemeine Chemie Volume 643, Issue 14 August 2017 Pages 895–902
DOI: 10.1002/zaac.201700049
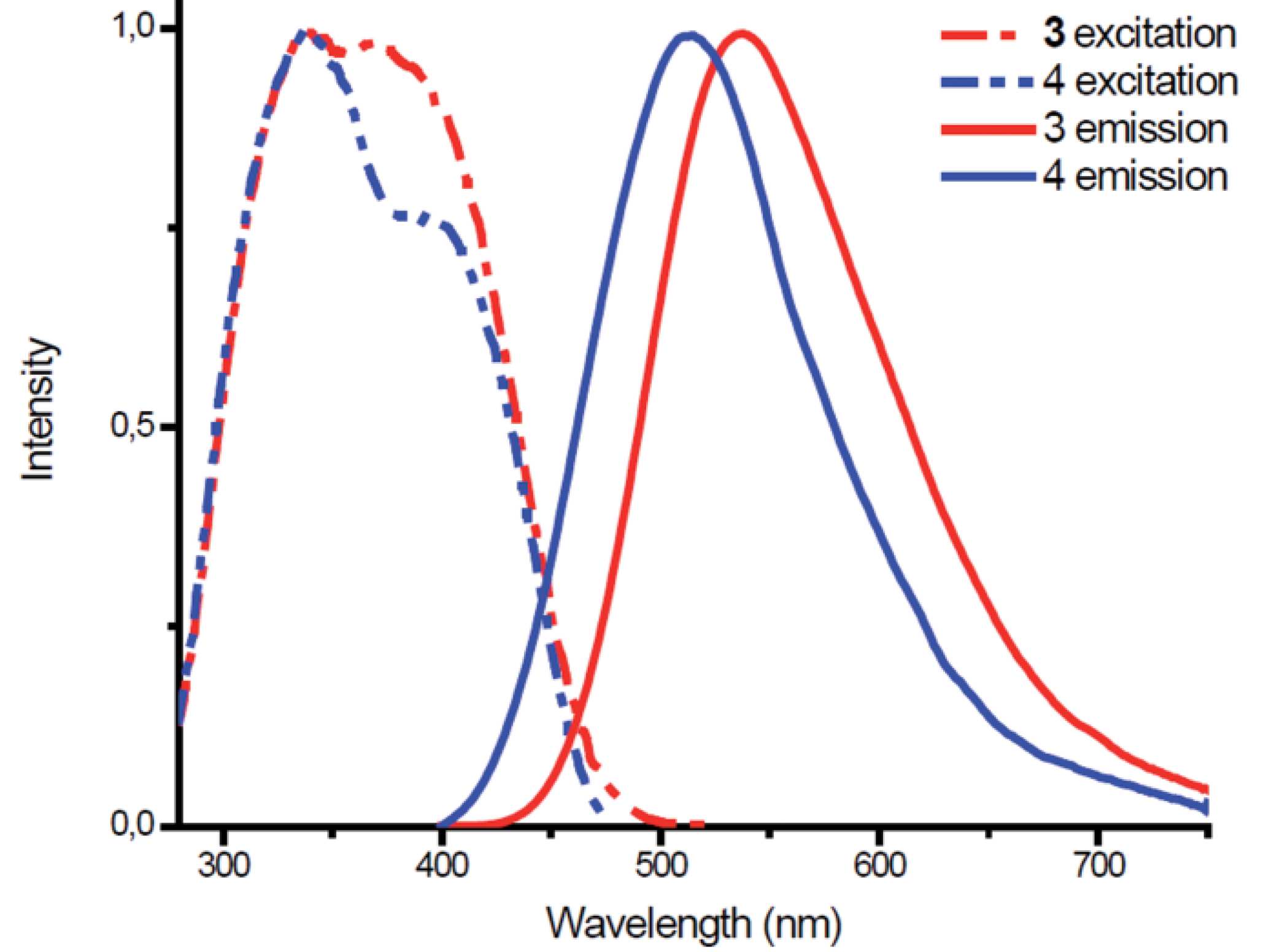
1,5-bis(R)-3,7-bis[2-(pyridine-2’_-yl)ethyl)-1,5-diaza-3,7-diphosphacyclooctanes 1 and 2 and their copper(I) complexes 3 and 4 were developed. The butterfly-shaped copper-iodide core and unusual P,N-chelate and P,P-bridged coordination mode of the heterocyclic ligand in the dinuclear complexes 3 and 4 were revealed. Complexes 3 and 4 display emission in green range of spectra, with lifetimes in a microsecond domain and quantum yields of luminescence in solid-state up to 38%. Thermochromic effects found for the phosphorescence of 4 in solutions are ascribed to rigidochromism.
- Информация о материале
- Просмотров: 1581
Journal of Fluorine Chemistry Volume 200, August 2017, Pages 18-23
Larisa B. Gulina, Valeri P. Tolstoy, Igor A. Kasatkin, Ilya E. Kolesnikov, Denis V. Danilov
Formation of oriented LaF3 and LaF3:Eu3+ nanocrystals at the gas − solution interface
Journal of Fluorine Chemistry Volume 200, August 2017, Pages 18-23
DOI: 10.1016/j.jfluchem.2017.05.006
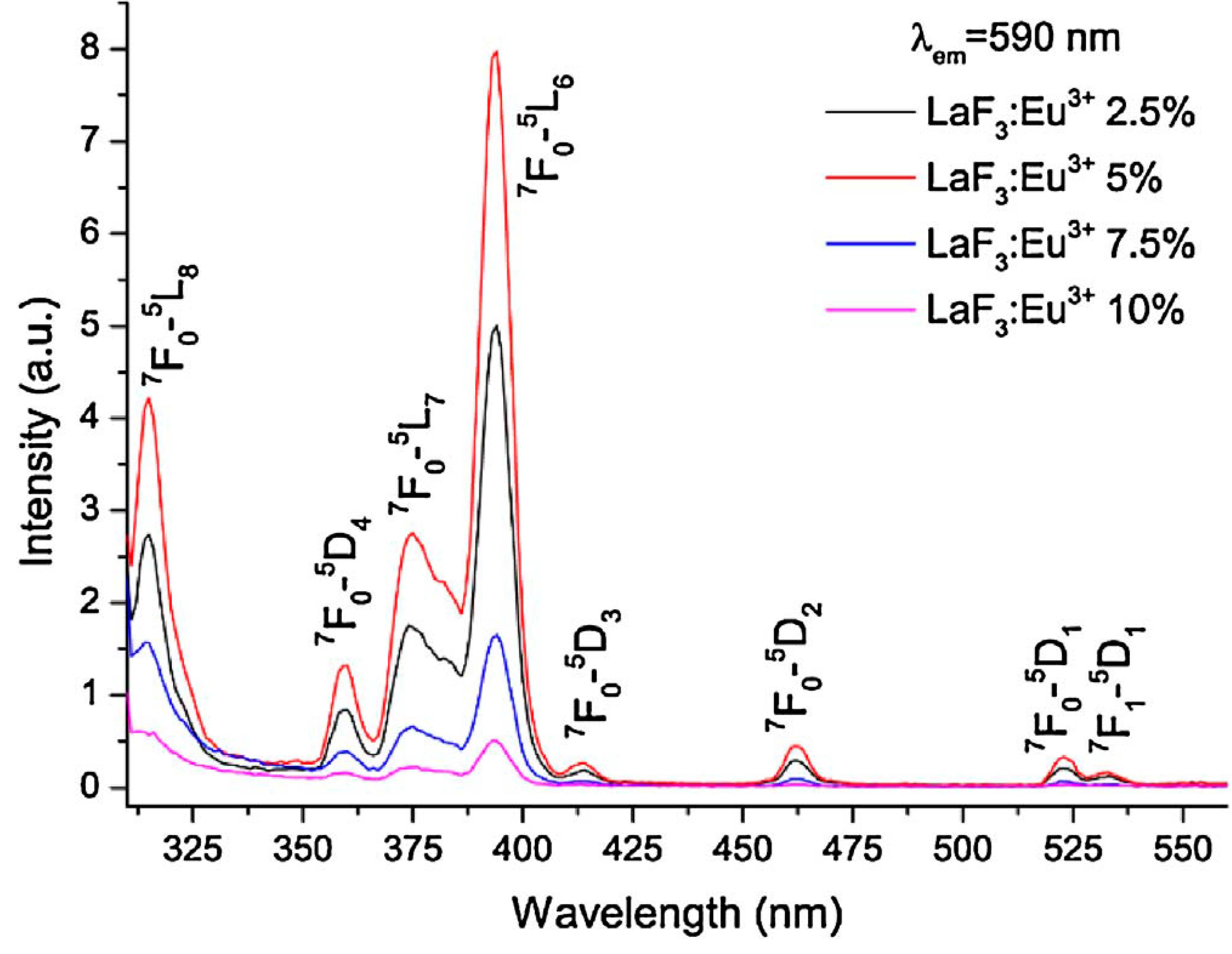
The LaF3 film formed by c-axis oriented uniform-size nanocrystals with diameter of about 400 nm was obtained on the surface of La(NO3)3 solution in 1 M HNO3 by interfacial reaction with HF in the gas medium. The effect of solution acidity on the layer morphology is reported. The film formed by LaF3:Eu3+ oriented nanocrystals with radial compositional gradient has been prepared in a similar way. The synthesized crystals were characterized by SEM, TEM, HRTEM, EPMA, TEM-EDX mapping, XRD analysis, Raman and luminescent spectroscopy. The LaF3:Eu3+ doped nanocrystals can potentially be used as luminescent material.




